A doctoral network
to develop advanced downstream processes and analytical tools for bionanoparticle manufacturing
Advancing the methods for characterization of bionanoparticles during production
Employing cutting edge analytical techniques
Enhancing safety and efficacy of bionanoparticle-based therapies
Developing more economical and efficient manufacturing processes
Current process

- Contains impurities and defective particles
- Slow off-line analytics
CAARE
- Faster time-to-market
- Cost-effective manufacturing
- Improved risk assessment
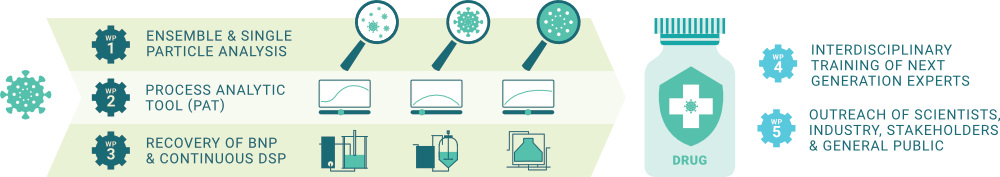
Our objective is to address the crucial aspect of biomanufacturing by developing new and intensified downstream processes to eliminate impurities and defective particles, ensuring a pure and potent therapeutic product.
14 Doctoral Candidates will be trained in advanced bioprocesses and state-of-the-art analytical techniques, providing them the skills needed to drive future innovations in biopharmaceutical manufacturing.
The network is composed of seven leading scientific beneficiaries and nine associated partners, six of whom are from industry.

